Patient and Public Involvement – what it means for you
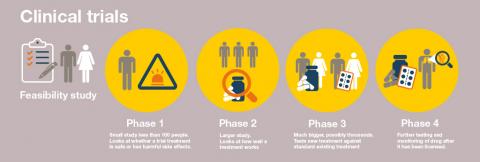
The Clinical Trials Accelerator Platform is an exciting initiative to help boost the number of clinical trials taking place in the UK and support equal access for everyone living with cystic fibrosis, and the Patient and Public Involvement (PPI) advisor will work with senior management at the Cystic Fibrosis Trust, ensuring that people with CF and families are represented at a strategic level.
It is a very exciting time in the world of cystic fibrosis: at long last there are transformational new drugs and therapies on the horizon – all that’s missing is how to boost the number of clinical trials and make it easier to take part. So, with the accelerator, the Cystic Fibrosis Trust and Cystic Fibrosis Foundation are pooling resources to increase the number of trials in the UK.
How will the process work?
We will invite CF clinics to get involved and will provide resources such as staff to ensure that trials can run. Crucially, we also need patient and public involvement on a local and national level – and that’s where you come in. PPI is important because without sufficient numbers of willing participants, trials cannot happen.
As PPI advisor, you can assist trials by:
- Giving researchers a sense of reality, saying what works and doesn’t work on several levels; from the practicality of organisational arrangements to how things feel. This can be as important as any medical outcomes.
- Helping make the language and content of information such as questionnaires and leaflets clear and accessible.
- Helping ensure that the methods proposed for any study are acceptable and sensitive to the situations of potential participants.
- Ensuring that the research uses outcomes that are important to the individuals or families who could be involved.
- Identifying a wider set of research topics than if professionals had worked alone.
- Increasing participation in research by making it more acceptable to potential participants.
Over time we want to build a network of people who will help steer the accelerator platform. A small patients’ group at each CF centre that joins the programme will support the clinical trial, and will link in with other groups to maintain high standards, help the Trust and participating clinics learn and help recruit trial participants.
As PPI Advisor, you will work alongside the PPI Coordinator, a full-time person based at the Cystic Fibrosis Trust, who will provide a bridge between the intended PPI network at CF centres, the wider CF community and the Trust.
The Advisor role is part-time on a consultancy basis, because we do not yet know how much or how little time the role will take – and this is to a large extent up to the post-holder. We can offer support to help organise tax and national insurance arrangements, if needed.
It is a flexible role, intended to be just a few hours a week, and that will mainly consist of online work reviewing documents, responding to email and so on. As time goes on there will be more telephone or skype work.
If you would like to apply, check out the full job description, and send us a CV with a covering letter saying why you are interested in the role.
