Trials Accelerator expands to support early phase clinical trials
Background
As the Trials Accelerator reaches its fourth year, we’re delighted to announce that we have recently created a specialist network of CF centres, trial coordinators and research nurses that will support early phase clinical trials.
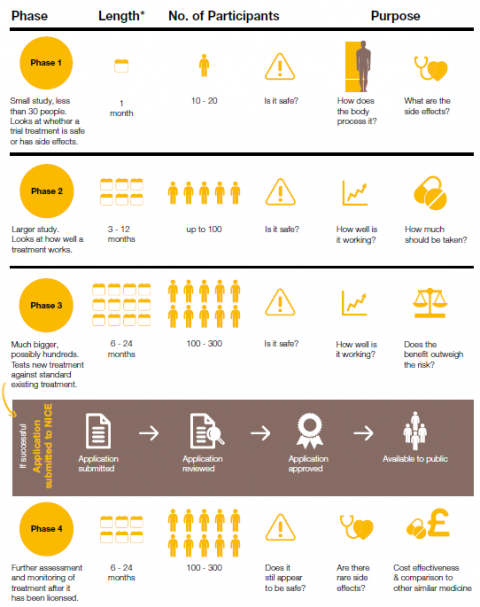
What are early phase trials?
Clinical trials often take place over several stages, or ‘phases’ Early phase clinical trials are one of the earliest stages of development for new cystic fibrosis (CF) therapies.
Early phase trials (sometimes referred to as ‘phase 1' trials) are usually when a new treatment is taken by a person with CF for the first time.
The main aim of an early phase trial is to:
- Check whether a new treatment is safe
- Monitor what effects a new treatment has on the body, and what, if any, side effects it may cause
Early phase trials are an essential stage in developing a new treatment. Without early phase trials, and without people with CF taking part in them, new treatments for CF like Kaftrio couldn’t have been developed.
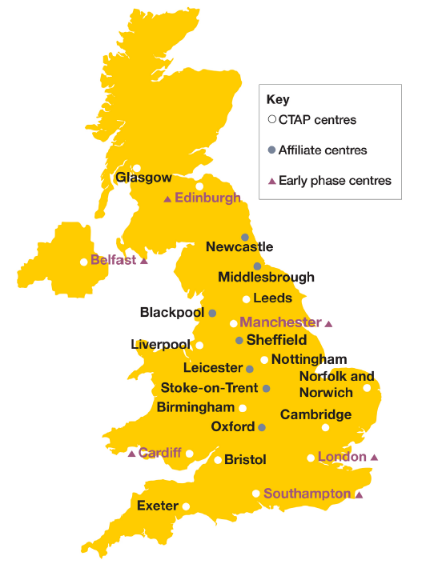
What is the new early phase network?
The early phase network brings together six Trials Accelerator centres to lead in the delivery of early phase trials for the UK. The centres are:
- Belfast Health and Social Care Trust (Northern Ireland)
- Cardiff and Vale Health Board (Wales)
- Manchester University NHS Foundation Trust (England)
- NHS Lothian, Western General Hospital (Edinburgh, Scotland)
- University Hospital Southampton NHS Trust Foundation (England)
- Royal Brompton and Harefield NHS Foundation Trust (London, England)
Each centre receives funding for an early phase trial coordinator or nurse, who will ensure early phase CF trials run smoothly and play an important role in helping eligible (suitable) people with CF to take part.
Why is the early phase network so important?
Whilst the Trials Accelerator has successfully supported many CF trials, few of these have been early phase trials.
This is because early phase trials require a great deal more resources in the way of research doctor and nurse time. The research team has to closely monitor participants of early phase trials to see how the treatment is being processed by the body, and to check for any side effects. Early phase trials may also require participants to stay overnight in hospital.
As a result of this, early phase trials in the UK have been limited in number. However, with the introduction of the new early phase network, a larger number of early phase CF trials will be able to open in the UK from 2022 onwards.

David has CF and has taken part in two early phase trials running at Southampton. Here, David talks about his experiences of taking part in the exciting early stages of research to develop new treatments for CF.
“I was invited to take part in a phase 1 trial by my CF research team in 2018 and again in 2019.
“Doing these trials is an amazing chance to further the development of CF treatments for the future.
“As Phase 1 trials are the early stages of developing a new treatment, it’s exciting but also a trip into the unknown. Reading the information packs and talking with the team answers any questions about procedures and risks, even if minimal.
“The nurses and doctors are with you the whole time during the visits. My trial even required overnight stays, but they were near all the time to monitor and ensure all was fine and I was comfortable and had everything I needed. The whole team are all fabulous and get through everything as smoothly, speedily, and safely as possible.
“If it’s worth doing, it’s worth doing as best as you can. Doing these trials is fantastic and feels like you are contributing to finding new and better options for CF treatments.”
Lorraine Hewitt is one of our new Trials Accelerator early phase nurses based at Southampton. Here, she tells us a little more about her role.
“As a nurse running a phase 1 study, I am responsible for ensuring that the extra safety requirements are met.
“All studies are slightly different and therefore the extra safety layers may vary, but ultimately, we plan for every eventuality when setting up a phase 1 study.
“For me, as the senior nurse for the study, my priority is ensuring that the patient is fully informed and happy to proceed with the study, understanding fully the risks, benefits, and what’s required from them to ensure their safety is maintained and that we obtain high quality accurate study data.”
If you’d like to learn more about taking part in early phase research, please contact your CF team or one of our Trials Accelerator early phase trial coordinators or nurses. You can also view our CF Trials Tracker for current trial opportunities.


Lorraine Hewitt
Early Phase Research Nurse
University Hospital Southampton NHS Trust Foundation

Benjamin Wilson
Early Phase Trial Coordinator
University Hospital Southampton NHS Trust Foundation

Natalie Hill
Early Phase Trial Coordinator
Manchester University NHS Foundation Trust

Jessica Matthews
Early Phase Trial Coordinator
Royal Brompton and Harefield NHS Foundation Trust
