Taking part in clinical trials
Find out more about taking part in clinical trials and what might be expected of you.
18 March 2020: Due to COVID-19 some cystic fibrosis (CF) clinical trials may be suspended with new trials postponed. Please see the relevant advice on the COVID-19 Q&A page.
Interested in taking part in a clinical trial but have some questions about how they work or what might be expected of you? Take a look at our information on taking part in a cystic fibrosis clinical trial or download our brand new clinical trials booklet.
While you're here, why not sign up to our clinical trials newsletter to stay up to date with the latest cystic fibrosis clinical trials updates?
Read on to find out more about taking part in clinical trials, including:
- How do clinical trials work?
- What are the different types of trials?
- Do all studies involve taking medicine?
- What are the phases of clinical trials?
- Can I take part?
- Should I take part?
- How can I take part?
- What will taking part mean for me?
- How long do clinical trials last?
- Are clinical trials safe?
- Will my information be confidential?
- Can I withdraw from a trial?
- FAQs - get the facts
- Patient and Public Involvement
- Useful websites
How do clinical trials work?
What are the different types of trials?
Clinical trials can be designed in different ways; controlled trials, randomised controlled trials and observational studies are all ways of investigating the impact of a treatment.
Most clinical trials looking at new treatments are controlled (comparative) - essentially they compare standard treatment, or no treatment, with a new intervention.
A randomised controlled trial (or RCT) is a controlled clinical trial as described above, but in this case participants are randomly assigned to either the treatment or control group. In some RCTs, the participants won’t know if they are receiving the new treatment or if they’ve been allocated to the control group where they will receive existing treatment or a placebo.
A placebo is an inactive substance, usually a capsule, pill or liquid, used in clinical trials to compare against a new medication drug. It is identical in appearance to the medication being studied but it has no medicinal action and is usually made of starch, sugar or salt.
Randomised controlled trials are generally thought to be the most accurate methods for testing the effectiveness of new interventions. This is because the randomisation lowers the risk of the trial results being skewed through bias, ie where researchers may (often unconsciously) select participants to receive either the intervention or existing treatment based on characteristics they display (for example age or disease severity). This can lead to inaccurate results.
To further reduce bias, RCTs may be blinded, or double blinded. In blind trials, the participant does not know if they are receiving the intervention or the existing treatment (or placebo), but the research team does. In double blind trials, neither the participants nor the research team know which participants have received the intervention, and which have received the existing treatment or placebo.
A trial in which both the researchers and participants know which groups are receiving which treatment is known as an open-label trial.
Do all studies involve taking medicine?
Some research studies may simply be observational. Observational studies aren’t designed to investigate a new intervention or treatment but instead are looking at different variables (such as mood or FEV1) over a period of time.
What are the phases of clinical trials?
Take a look at the different phases of clinical trials and how they might involve you.
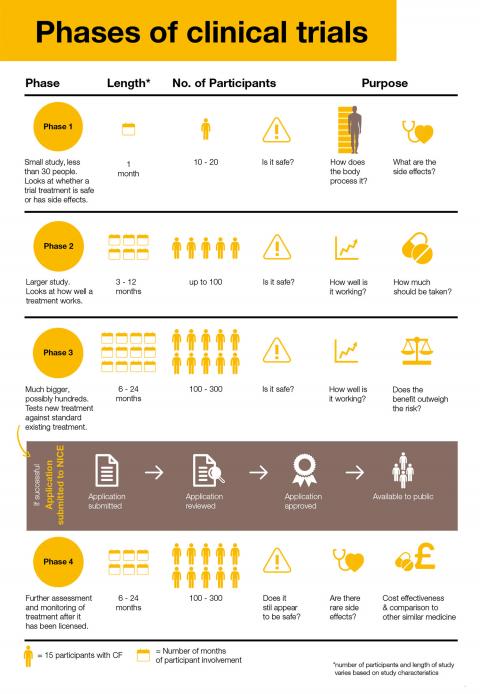
Can I take part?
Anyone can potentially take part in a clinical trial, but whether or not you are suitable to will depend on the eligibility criteria for each specific trial. A screening visit will help determine if the trial is suitable for you by checking your health and medical history against the inclusion and exclusion criteria.
The screening process should be fully explained to you beforehand and will not take place until after you’ve given your consent to take part in the trial. Eligibility criteria vary from trial to trial and are typically based on characteristics such as:
- Age
- CF genotype
- Severity or stage of illness
- Pregnancy
- Treatments currently in use
- What other medical conditions may be present
Being told you’re not eligible to take part in a particular clinical trial can understandably be very disappointing, however you may find you are eligible to participate in a different trial. It’s really important that clinical trials have strict eligibility criteria in place to make sure the trial is conducted as safely and accurately as possible.
Should I take part?
Deciding whether or not to take part in a clinical trial is a personal decision; you should never feel pressured to take part. People with CF who have participated in cystic fibrosis clinical trials told us that it’s really useful to get as much information as possible about the trial, so you can make an informed decision.
You should take as much time as you need to decide whether or not to take part. You may also find it useful to talk about it with your family and friends, especially any possible impact on your personal life, work/school and family commitments.
It is important to be aware that you would not necessarily receive a new treatment by participating in a trial – you may receive standard treatment or a placebo, but this still often contributes to a positive experience by helping identify the best treatment for yourself and others.
Taking part in research can be very rewarding. There are many reasons for getting involved in research:
- Understanding your condition better.
- Taking a more active role in your healthcare and decisions that affect your treatments and quality of life.
- Feeling good about helping advance medical knowledge.
- Helping researchers to develop new treatments for the benefit of future generations.
- Benefiting from regular follow up meetings when involved in a clinical trial. This can mean that health problems may be found earlier.
- Receiving treatments that are not yet widely available.
How can I take part?
Thinking about taking part in a clinical trial is a big decision, and it’s important that you have all the information you need to make an informed decision. It might be helpful to start by finding out what trials are taking place, and there are a number of ways you can do this.
CF Trials Tracker
We have created the UK’s only CF-specific clinical trials database, the CF Trials Tracker. This database lists cystic fibrosis clinical trials and research studies open to recruitment with details about each trial and contact details of who to contact for more information. Head over to the Trials Tracker to search and apply for new clinical trials for cystic fibrosis.
Your CF team
Your CF team may already be proactive in discussing taking part in cystic fibrosis clinical trials with you. If not, raise it and let them know that you would like to be kept up to date with what trials are taking place, and potential opportunities to participate, whether locally or at other CF centres you may be willing to travel to. You may have found something of interest on the Trials Tracker to start this conversation.
Remember that just having a discussion about taking part in a trial doesn’t mean you are obliged to take part, and you should never feel under any pressure to do so. Whether or not you decide to take part will have no impact on the care you receive from your CF centre or clinic. You can also withdraw from a trial at any time, without giving any reason or it affecting the regular CF care you receive.
What will taking part mean for me?
Clinical trials take many different forms, and what is required will vary greatly depending on what is being researched.
Many trials involve additional ‘research visits’ to your CF centre (or the CF centre running the trial), where questionnaires about how you’ve been feeling will be completed and any necessary clinical measures taken ie blood test and spirometry. Researchers will also look out for any side effects and ask about any positive or negative changes you have experienced.
You will be monitored regularly during the trial and for follow up appointments. Sometimes this means going to your CF centre more often than you would normally.
There is always the risk that the trial treatment could result in some unwanted side effects. However, the extra attention you receive during clinical trial participation means that any changes in your health, whether related to CF or to the treatment you are having, is likely to be picked up sooner than if you weren’t in a trial. Some people with CF have said that they found the extra focus on their health made them think more about their condition, which could be a good or bad thing.
How long do clinical trials last?
Although trials can run for many months, or even years, it doesn’t mean that you would be expected to participate for this duration! There are several reasons why trials can be lengthy:
- It can take a long time to recruit enough people to take part in the trial (even a small number of people with a rare mutation).
- It may involve giving a treatment over a long period of time.
- It may be important to follow up with patients over a long period of time to get a reliable picture of the long-term effects of a treatment.
Understandably, for those who stand to benefit from a new treatment in trials, and particularly for those with life-shortening conditions, the length of the trial process can be frustrating.
Speak to the trial team to get a full picture of how long you would need to take part, and how often you will need to visit the CF centre for research visits.
If a trial is being conducted at your own CF centre, appointments may be scheduled to take place before or after your routine clinic appointments, to try and minimise the number of visits you have to make. However, this isn’t always possible, and while trials generally aim to be flexible, some appointments may be needed during school or work hours. Inevitably, trial participation can sometimes present challenges in juggling the trial with your personal life, employment, education or other commitments.
Are clinical trials safe?
Understandably, safety is often a key concern people have about clinical trials. Clinical trials are carefully controlled, regulated and organised to minimise risk to participants. Serious side effects are very rare and clinical trials are now safer than ever.
New medications will have been checked using human and animal cells, before ever being administered to humans. However, because some clinical trial interventions are investigational, there is always a small possibility of unpleasant or potentially serious side effects of any new treatment. It’s worth remembering that not all trials are for new medications – some trials involve commonly prescribed medications to see if treatments effective for other conditions are also effective in cystic fibrosis.
During the informed consent process, you should be told everything that the researchers know about any possible risks and side effects so that you can make an informed choice about whether to participate.
Every trial has an independent Data Safety Monitoring Board (DSMB), and if anything happens during a trial, such as a bad reaction, it is immediately reported to the DSMB. The board will stop a trial if there is any doubt about the safety of the treatment.
Will my information be confidential?
Yes. If you agree to take part in a clinical trial, any information that is collected about you will be kept confidential. The researchers cannot tell anyone that you are taking part in the trial without asking you first.
Once the trial has finished, the results may be published in a scientific journal, or at a conference, but no identifying information about participants in the trial would be shared.
Can I withdraw from a trial?
You can withdraw from the trial at any time, and without giving a reason. This will not impact on your care in any way.
Depending on the type of trial, the participant may be given advice on how to withdraw from the trial safely, for example when new medicines are being investigated.
Get the facts
It’s really important you have all the facts you need to make an informed decision about whether to participate in a clinical trial. People with CF have helped us to put together a list of questions that it might be worth considering before you decide to join a clinical trial.
Patient and Public Involvement
Patient & Public Involvement (PPI) is integral to our Clinical Trials Accelerator Platform and differs from participation in a trial. If you want to get involved and have your say in how research for CF is conducted, head over to our PPI page.
To register your interest in getting involved or to receive news updates, email [email protected].
Useful websites
- Generation R is an organisation dedicated to involving young people in the design and delivery of research.
- Health Talk has a section dedicated to people’s experiences of clinical trials.
- For guidelines on research involving children, take a look at this ethics guide from the Medical Research Council.
Meet the team
There are a number of different professionals involved in making sure clinical trials are as successful as possible.
Informed consent
Informed consent is there to make sure you find out everything you need to know about a trial and how you will be involved.
Trials for young people
Take a look at the resources available for young people involved in clinical trials.
Clinical trials booklet
Our clinical trials booklet is full of useful information on taking part in trials.
